Some Important Compounds of Transition Metals
Some Important Compounds of Transition Metals: Overview
This topic covers concepts, such as, Important Compounds of d-Block Elements, Potassium Dichromate, Reaction of K2Cr2O7 with Conc. Cold H2SO4 & Reaction of K2Cr2O7 with Conc. Heated H2SO4 etc.
Important Questions on Some Important Compounds of Transition Metals
A compound which is a strong oxidizing agent and has orange coloured crystal. It is used in the preparation of azo compounds. Identify the compound:
On oxidation of by in neutral aqueous medium, the oxidation number of S would change from:
On complete oxidation of in acidic medium, the oxidation state of Cr will change from:
The solution of potassium dichromate is prepared from which of the following compound and how its colour changes with change in pH:
The number of moles of that will be needed to react with one mole of sulphite ion in acidic solution is:
The following steps are involved in the manufacturing of potassium dichromate:
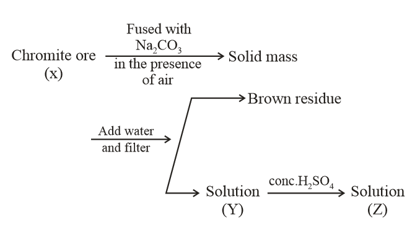
What is the difference in the oxidation number of between X and Y?
What is the colour change when solid potassium dichromate is heated with concentrated sulphuric acid?
Solid potassium dichromate when heated with concentrated sulphuric acid gives which of the following product?
when reacts with cold conc. gives red crystal of
Which of the following product is not formed when potassium dichromate reacts with cold sulphuric acid?
The fusion of chromite ore with in air gives a yellow solution upon addition of water. Subsequent treatment with produces an orange solution. The yellow and orange colours, respectively, are due to the formation of
What is the melting point of potassium dichromate?
Which of the following statements is correct about tetrahedral manganate and permanganate ions?
Which of the following statements is/are false?
A solution of is reduced to various products depending upon its .
At , it is reduced to a colourless solution ().
At , it forms a brown precipitate ().
At , it gives a green solution ().
Then and , respectively are
Which of the following sodium salts, on heating with solid and concentrated , gives red vapour?
Which of the following species has oxygen in oxidation state?
How many peroxy linkages are present in ?
Treatment of alkaline solution with solution oxidizes iodide to:-
The above reactions give us permanganate ion from potassium oxide. The no. of manganese dioxide in the product side for both the reactions are:
